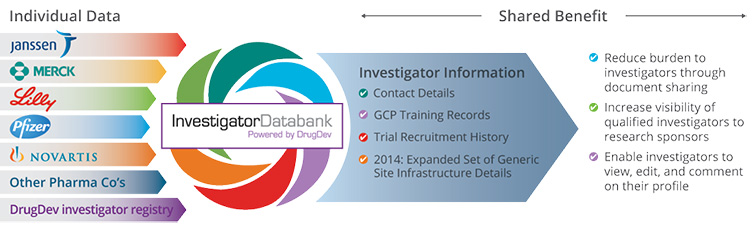
Janssen led the establishment of the Investigator Databank as part of a novel cross-industry collaboration involving Merck, Eli Lilly, Pfizer and Novartis, with more companies to come, that aims to reduce the administrative burden for investigators and increase the visibility of qualified investigators to research sponsors. The databank, hosted by DrugDev.org, is a one-stop repository where key information about clinical trial sites, such as infrastructure details, Good Clinical Practice training records, trial participation and recruitment history is shared. The ability to easily access this information reduces the time-consuming and redundant administrative work involved in identifying and interacting with appropriate clinical trial sites.
- Databank Query System: A query tool that enables sponsor companies to search for specific investigator and/or trial site information
- A website providing investigators with a window into the Investigator Databank, enabling them to view and edit their profile, comment on studies and upload training certificates and other documents. This will increase the accuracy, transparency and investigator level of control of the information listed
Find out more about the Investigator Databank at www.investigatordatabank.org
If you are an investigator interested in receiving a link to opt-in to the Investigator Databank, please contact the Investigator Databank
All third party trademarks used herein are trademarks of their respective owners.



