Patient Engagement - Treatment Lifecycle
Dr Paul Janssen, founded Janssen, an organisation dedicated to creating medicines that meet unmet patients needs. Patients are at the centre of all we do. As Dr Paul often said, “There is so much more to be done; patients are waiting.” For us this is more than a reminder – it’s an urgent call to action. And it’s on this foundation that Janssen Australia and New Zealand continues to ensure that the patient is at the centre of everything we do. Each of us. Each and every day. We continuously look for ways to further ensure that all our decisions are made with the impact on our patients in mind.
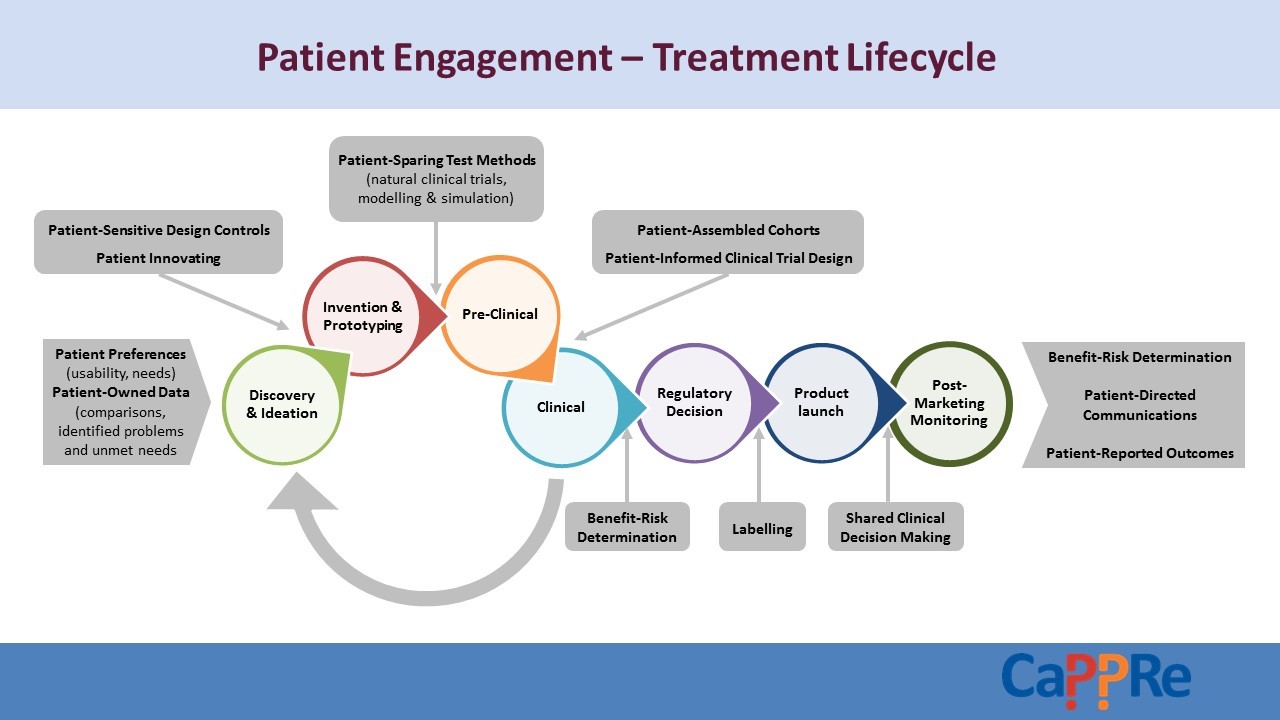
The development of a medicine is a process that calls on the expertise and input of many, all working towards the common goal of improved outcomes for patients. As the diagram illustrates, patients are both the origin and the destination in this process, so it’s vital that the patient perspective is represented in the medicine lifecycle. Here are four examples of how we partner with our patients every day to keep them truly at the centre of all we do:
1. Improving patient engagement in clinical trials: Clinical trials are a key step in the development of a new medicine. We’re currently focused on incorporating the direct patient voice earlier in our medicine development process by using patient perspectives to inform protocol design. We’re listening to patients and seeking out their self-expressed preferences about research design and implementation so we can understand and address barriers to clinical trial recruitment and participation, and we’re promoting accessibility to trial information, results, and data.
For patients living outside of Australia’s major metropolitan centres, access to clinical trials is hampered by many barriers including distance and cost of transport. To help address these issues, we are a proud member of the consortium supporting the Pilot Implementation of the Australian Tele-Trials Model. This project aims to use tele-health strategies to increase clinical trial access for cancer patients closer to home.
2. Mapping the patient journey: Our job is not just to treat diseases – it’s to treat people. To treat our patients as whole people, we need a comprehensive understanding of their lived experience from end-to-end. Mapping the patient journey through the lens of both patients and healthcare professionals helps us to better understand:
- The current experience for patients at key points in their journey.
- Underlying motivations impacting a patient’s behaviour and response to different aspects of their disease and treatment.
- Pain points and incremental improvement opportunities within the health system.
- Potential solutions to improve the experience of both patients and healthcare professionals.
3. Innovative Partnerships
We know that treating disease goes beyond just medicine; that managing physiological impacts of disease is only half the battle. Many consumers – particularly those living with #MentalIllness or chronic conditions – experience psychosocial impacts as a result of their physical disease, such as stigma, social isolation, emotional distress, and stress on relationships.
We are working with healthcare providers and government agencies to understand broader challenges and create innovative solutions to support consumers living with complex mental illness.
4. Patient Voice Initiative:
Being patient centric is about much more than our own processes and projects, it’s also about working towards a health landscape that is truly patient centric. It means collaborating with those outside our organisation in an effort to keep patients at the centre of the system in which we operate.
In 2015 we helped establish the Patient Voice Initiative (PVI) in Australia. This initiative brings together patients, heath consumer organisations (patient groups), academia and government to discuss how to gain the patient perspective on the value of medicines. The initiative has generated an ongoing dialogue about how the Australian health technology assessment (HTA) system can better incorporate the view of patients.
The work done locally was also recently discussed at the Health Technology Assessment International (HTAi) 2017 Annual Meeting which included specific workshops and sessions on Patient and Citizen Involvement in HTA. Jo Watson, Deputy Chair and Consumer Representative of Australia’s HTA body (Pharmaceutical Benefits Advisory Committee) also attended the HTAi meeting. Jo and Jessica Bean, Chair, PVI, have committed to exploring options for continuing to work together to ensure patient perspectives are front and centre of the HTA discussion in Australia.
As a pharmaceutical company with innovation in our DNA, we have always focused on the needs of our patients. It’s a shared responsibility across every area of our business to continue to support our culture of putting the patient at the centre of everything we do and, in doing so, improving outcomes.
CP-01370 August 2017
