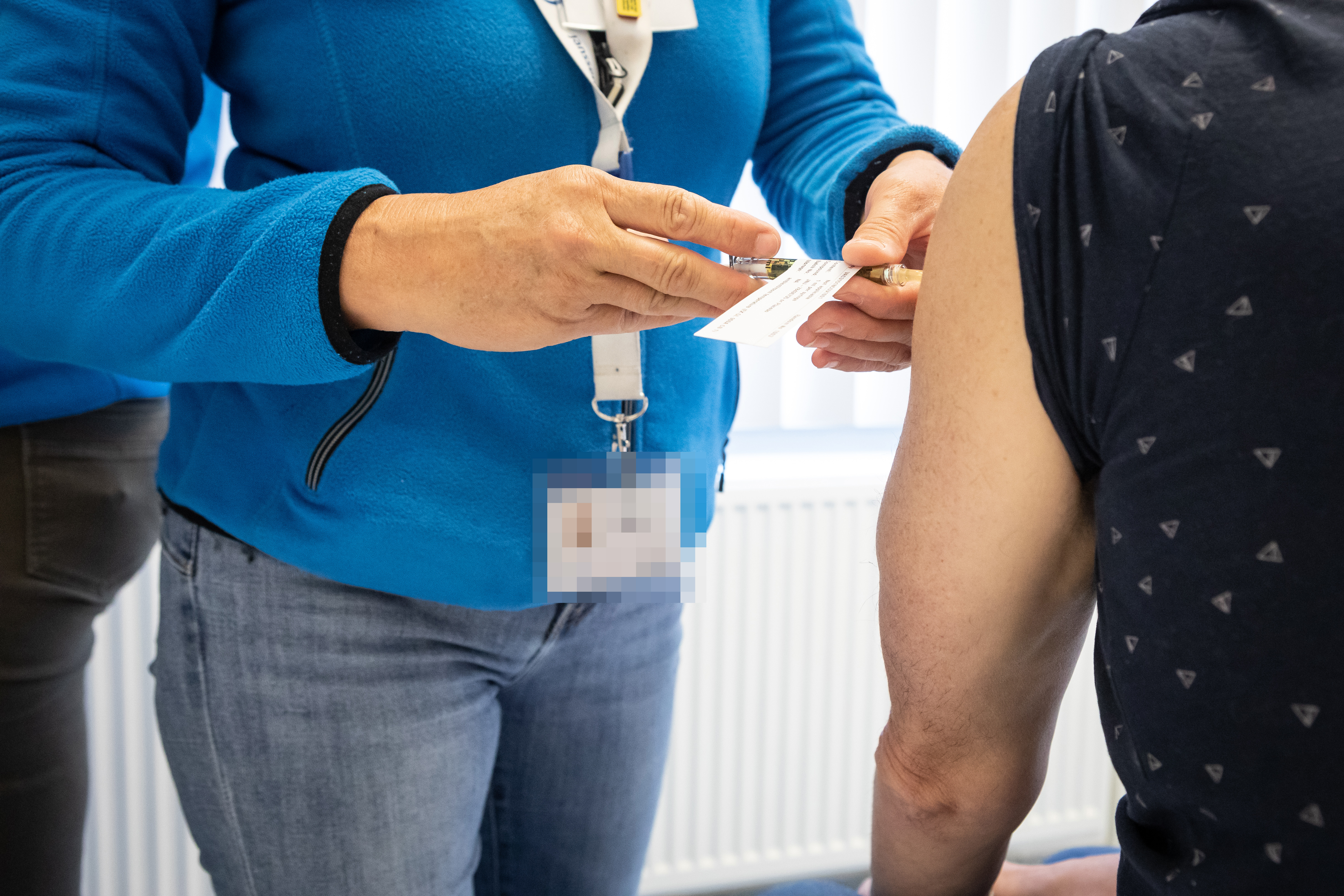
Clinical trial COVID-19 vaccine candidate underway
The road to the vaccine
From the moment the genetic code of the coronavirus became known at the beginning of this year, Janssen researchers in Leiden and Beerse have been working hard to develop a vaccine against COVID-19.
In the pre-clinical development phase, the most promising candidate from a dozen vaccine candidates was selected at the end of March. This vaccine candidate now has to go through the further clinical research steps. For example, we are developing a vaccine that can be produced in large quantities and then used worldwide, if it proves to be safe and effective in clinical trials.
Clinical trials
The study now underway belongs to the first clinical research phase (phase 1/2a) that must be completed before a vaccine can be marketed. A total of more than 1,000 healthy volunteers in Belgium and the United States are taking part in this study.
We are making every effort to develop a safe and effective COVID-19 vaccine to protect people around the world from this pandemic. After today, we are an important step closer to achieving that goal
Paul Stoffels
Vice Chairman of the Executive Committee and Chief Scientific Officer, J&J, who witnessed the vaccination of the first healthy volunteers
In the phase 1/2a clinical trial, we investigate the safety profile and immune response of the vaccine candidate. Only after the safety of the vaccine has been established may the vaccine be administered more widely. In the second phase of the clinical trial, the vaccine candidate will be studied in a group of healthy volunteers. Depending on the results of the phase 1/2a study, the second phase will start in several clinical centres in the Netherlands, Germany and Spain. Provided everything goes well and subject to the approval of the regulatory authorities, the Phase 3 clinical trial will start in September in those countries and regions where the coronavirus is active and therefore in the population for which the vaccine is intended. At this stage of the clinical trial, the safety and efficacy of the candidate vaccine will be assessed on a large scale.
Shortened timelines
Under normal circumstances, the completion of all phases of clinical trials takes about 7 years. 1 However, these are exceptional circumstances. That is why we are currently making every effort and expect to receive the first results sooner. The shortened timelines are possible because of our extensive expertise in vaccine development and because we use the same technology we used to develop our vaccine candidates against Ebola, Zika, RSV and HIV.